Homepage
NEW!!! 10/12/07
Sustainable Agriculture Technologies:
KEYS TO UNDERSTANDING SOIL'S & SOIL TESTING...
For SUSTAINABLE SOIL MANAGEMENT
Dr. Allen Stout - 1972, Updated 2007
 A thin fragile layer of living Topsoil, varying from a few inches to a few feet thick, covering much of the Earth's surface is all that stands between humanity and starvation. The rise and fall of great civilizations have been determined by how the soil has been managed or exploited (see "World Crisis in Agriculture" and "Conquest of the Land Through 7,000 Years").
A thin fragile layer of living Topsoil, varying from a few inches to a few feet thick, covering much of the Earth's surface is all that stands between humanity and starvation. The rise and fall of great civilizations have been determined by how the soil has been managed or exploited (see "World Crisis in Agriculture" and "Conquest of the Land Through 7,000 Years").
Soil scientists define soil as a mixture of mineral and organic matter, formed from weathered rock by action of climate and living organisms, that is capable of supporting plant life (15, p. 1-2). The question for those who manage the land is, "How can you know the proper fertilizer and management needs for sustainable management of your soils?"
The technical knowledge of soils and soil testing methods can, if properly used, be a valuable aid in determining the specific condition, and the fertilizer and sustainable management needs of the soil, and help you to avoid the costly mistakes of trial and error fertilizer applications.
CONTENTS:
Introduction
Return to Top
There are basically two concepts regarding fertilization. One is that fertilizers are soil food and should be used to feed the soil, which, according to natural science, allows the biological and colloidal mechanisms of the soil to feed the plant.
The other concept, promoted largely by the fertilizer industry, is that fertilizer is a plant food to be used to feed the plant directly, often ignoring and "bypassing" the biological and physical properties and functions of the soil (14, p 223). Clearly, there is a need for better understanding of soil and the principles governing soil fertility and plant nutrition.
Ironically, as we apply more and more chemical fertilizers and pesticides to our land than ever before, we have become more and more dependant on high-cost non-sustainable chemical technologies, and the problems are in general worsening. [Note: These originated largely from war inductries and technologies that exploded during World War II. N-P-K fertilizers were from the chemical ammuitions factories and pesticides were developed for jungle warfare]
Side effects of the Chemical Revolution in agriculture have been pollution of our planet, depletion of non-renewable resources, and skyrocketing increases in health care needs and costs.
The pollution of our soils, water, air and foods following WW II is now beginning to take a high toll, reaching a crisis worldwide, in the effects on health and the environment (see "Food Quality & the Health Crisis"). Cancer rates began skyrocketing as chemical use increased and epidemics of metabolic, degenerative and autoimmune diseases, and new or reemerging infectious diseases have become the new plagues.
An important factor causing many of these problems, little understood by most, is improper and imbalanced fertilizer treatments (see "Cattle Nurtition & Health Program". Part of these problems arise from the misunderstanding of soil and crop requirements and what fertilizers are really needed, how they should be used and for what purpose.
Soils vary greatly in their composition and condition. Fertilizers needed by one soil may be detrimental or even toxic in another. Yet most fertilizers applied today are applied with little regard for the SOIL's fertilizer needs, capacity, or balance.(14, p 223)
Soil testing services are available from many state and private testing laboratories, and soil testing kits are available for the do-it-yourselfer. But the accuracy and actual value of the test results of various labs and kits vary considerably, some even being worthless or sometimes misleading and detrimental (12a, p 178,180; also 4, p 302-04). It is necessary to understand something about soils and soil testing, plant nutrient requirements as well as soil and crop management principles to properly understand and interpret the results of soil tests. Some of these factors will be discussed in the following paragraphs.
Crop Requirements
Return to Contents
For maximum production and quality, plants generally require sunlight, a balanced combination of readily available minerals, water, oxygen, carbon dioxide and various organic substances, plus suitable environmental conditions and soil for strong root development.
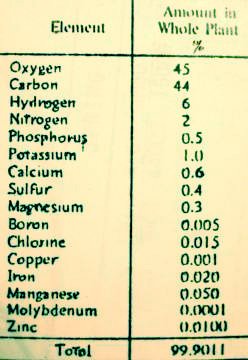 Twenty elements are presently known to be essential for the growth of plants, and the importance of others is beginning to be recognized (9, p 105; also 4, p 4). Not all are required by all plants, but all are necessary to some plants. Carbon, hydrogen, and oxygen are derived from air and water; nitrogen from air and soil; and phosphorus, potassium, sulfur, calcium, magnesium, sodium, iron, manganese, boron, copper, cobalt, zinc, molybdenum, vanadium, chlorine and silicon from the soil (4, p 4). Plants also contain iodine and selenium (elements essential to animals) and many other elements whose functions in plants are not totally known (12c, p 80; also 9, p 533-34; & 4, p 11).
Twenty elements are presently known to be essential for the growth of plants, and the importance of others is beginning to be recognized (9, p 105; also 4, p 4). Not all are required by all plants, but all are necessary to some plants. Carbon, hydrogen, and oxygen are derived from air and water; nitrogen from air and soil; and phosphorus, potassium, sulfur, calcium, magnesium, sodium, iron, manganese, boron, copper, cobalt, zinc, molybdenum, vanadium, chlorine and silicon from the soil (4, p 4). Plants also contain iodine and selenium (elements essential to animals) and many other elements whose functions in plants are not totally known (12c, p 80; also 9, p 533-34; & 4, p 11).
Approximately 95% of these plant nutrients come directly from the air and water. Another 1 to 2% (nitrogen) comes from the air and soil organic matter. The small but vital remaining 3 to 4% must come from the available mineral and organic matter reserves of the soil. Most plants require the same basic nutrients, but they often differ considerably in the relative amounts of these nutrients required and the soil conditions they can tolerate. For example, blueberries, alfalfa and rice all require the same general soil elements as well as air and water (4, p 1), but blueberries generally tolerate acid soils very low in both available alkaline minerals and water, while alfalfa generally requires a very fertile alkaline soil with abundant moisture. On the other hand, alfalfa and rice both have a high water requirement, but alfalfa must have good aeration of the soil with the moisture, whereas rice does well when the soil is flooded with water to control weeds. Blueberries and rice must both obtain all of their nitrogen from the soil, but alfalfa can obtain a large portion of its nitrogen from the air through nitrogen fixing bacteria in root nodules.
Interestingly, weeds often thrive in poor infertile or imbalanced soils. Though a plague to farmers, ecologists have noted their value in building fertile soils. It should be recognized that soil fertility is only one of the major factors important in crop production.
Crop yield, both quantity and quality, is a function of four major factors:
- the soil upon which crops are grown,
- the crop variety and genetics,
- climatic conditions,
- and the management practices followed (10, p 57).
A sure way to test the productivity of a soil is to grow plants on it (4, p 3). Every crop has a built-in or bred-in potential of production (9, p 662). This potential is seldom reached because there may be one or more limiting factors holding back production.
For more information on Crop Requirements, see Table of Crop Requirements
Sustainable Soil Management Goals
Return to Contents
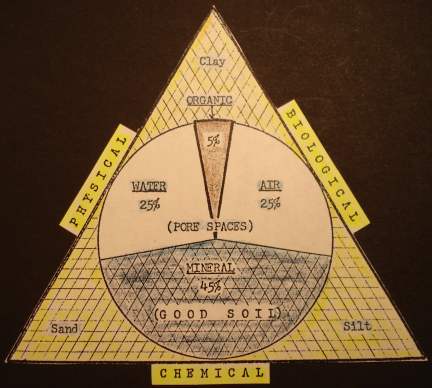
Truly fertile soils consist of minerals, organic matter, water, air and living organisms (12b, p 17). But the proportions of these components and the resulting fertility of different soils vary considerably. Three key aspects of long term sustainable soil management involve managing the soil's 3 main components:
- The mineral-chemical component,
- the physical structure with pore spaces for air and water,
- and the organic-biological component.
Mineral-Chemical
The mineral-chemical base of different soil profiles will vary considerably because of the different parent rock materials the soil is formed from. Also climatic conditions have a major effect on the mineral-chemical component. Minerals leach in high rainfall areas resulting in depleted low pH soils, and in dry areas soils will accumulate alkaline minerals in the topsoil causing high pH and sometimes toxic conditions. Normally only a small portion of the parent minerals will be in chemically available forms that plants can readily utilize. Availability will depend on fineness of the parent minerals and chemical and biological functions in the soil. Finely ground parent rock minerals may need to be added to soils that lack essential minerals. Standard soil tests do not measure the soil's unavailable mineral reserves.
Physical Structure & Tilth
Physical structure and tilth is determined by mineral composition and texture and the organic matter content and biological activity. A major purpose of tillage is to effect the soil condition to improve water absorption and drainage and aeration. Improper tillage practices often have the opposite effect on long term soil condition. A soil penetrometer is commonly used to determine the condition of soil tilth and detect hard pans, etc.
Organic Matter & Biological Activity
The soil's organic matter (humus) level and biological activity are key components to building and maintaining fertile topsoil in a sustainable soil management program to improve soil structure and tilth and optimum availability of soil nutrients. Ideally one should try to build and maintain a 5% or more level of organic matter in the topsoil. The deeper the topsoil profile, the higher the productive potential.
The fertility and condition of the land is commonly determined by observations of the physical properties - topography, profile, color, texture, structure, odor, determination of numbers and various species of soil micro-organisms, earthworms, etc.; observations of growing plants (and weeds) types and the growing crops, their health and resistance, production yields, deficiency symptoms, etc.; quality and effects on health and production of animals; chemical soil analysis; plant tissue tests; trial applications of fertilizer materials; etc. (12a, p 172).
The objective of a sound long teerm sustainable soil management program is build and maintain an abundance of all the essential nutrients in the proper balance for efficient maximum production of healthy, disease and insect resistant, high quality crops and food. A deficiency or toxicity of any one or combination of elements or conditions necessary for plant growth may limit the production, quality or health, and resistance of the plants and those consuming them. (see "Cattle Nurtition & Health Program". Many of the symptoms of deficiencies and toxicities can, with experience, be recognized in the field (14 p 172; also see 11 whole book). The deficiency diagnosis can then be checked by soil and plant tests and/or trial application of fertilizers in the field.
Soil Composition
Return to Contents
The mineral portion of the soil accounts for only 3 to 4% of the plant, but its importance to soil fertility and plant nutrition is much greater. 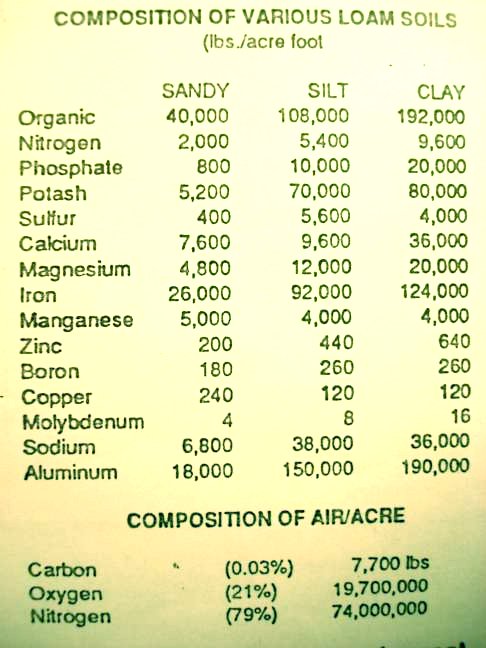 The mineral portion consists of sand, silt, clay and larger rock particles and the soluble ionized mineral elements in the soil solution and attached to the soil colloids. The mineral particles give a soil its characteristic texture and serve as a storehouse for most of the soil's mineral reserves. However, very little of these reserves are available to plants. For example, an average acre foot of a silt loam soil may contain 70,000 pounds of total potash, but depending on the mineral types, only 100 to 600 pounds may usually be in a form available to plants (12h, p 101).
The mineral portion consists of sand, silt, clay and larger rock particles and the soluble ionized mineral elements in the soil solution and attached to the soil colloids. The mineral particles give a soil its characteristic texture and serve as a storehouse for most of the soil's mineral reserves. However, very little of these reserves are available to plants. For example, an average acre foot of a silt loam soil may contain 70,000 pounds of total potash, but depending on the mineral types, only 100 to 600 pounds may usually be in a form available to plants (12h, p 101).
The soil's mineral reserves are made available by the slow processes of weathering and by the much more rapid biochemical processes of plant roots and soil microorganisms. Soluble minerals, unless held by the electrical charges of the soil colloids, may be lost downward through leaching, or in hot dry conditions accumulate near the surface possibly resulting in salt toxicity (14, p 226). The soil's capacity to hold the cations (calcium, magnesium, potassium, etc.) in the available or soluble non-toxic state is dependent upon the soil's cation or base exchange capacity (CEC), sometimes called total exchange capacity (TEC) (12c, p 82).
Soil Cation Exchange Capacity (CEC) & pH
Return to Contents
A soil's Cation Exchange Capacity is considered the best single index of potential soil fertility (4, p. 59). 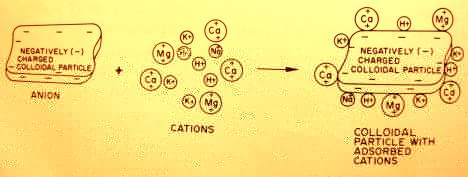 CEC is a property of the soil colloids (clay and humus content), which have a negative charge to hold positively charged cations (Ca, Mg, K, Na, H and trace mineral cations).
CEC is a property of the soil colloids (clay and humus content), which have a negative charge to hold positively charged cations (Ca, Mg, K, Na, H and trace mineral cations).
CEC is generally calculated by measuring the amounts of exchangable calcium, magnesium, potassium, sodium and hydrogen a soil will hold (in milliequivalents (ME) per 100 grams of soil).
Cation exchange capacity (CEC) range of soils:
- Sandy soils may range from 0 to 5 ME
- Loams from 5 to 15 ME
- Clay loams from 15 to 30 ME
- Clay soils over 30 ME
- Humus from 100 to 300 ME (4, p. 59-60; 15, p. 154).
For practical application the ME's of the soil CEC must be converted to pounds of the mineral cations an acre of soil 6 inches deep ( 2,000,000 lbs of soil) can hold on the soil colloids (CEC). The following gives the amounts of each cation an acre of soil can hold (when totally saturated) for each 1 ME of CEC:
- Calcium (Ca) = 200 ppm (parts per million) or 400 lbs/acre
- Magnesium (Mg) = 120 ppm or 240 lbs/acre
- Potassium (K) = 390 ppm or 780 lbs/acre
- Sodium (Na) = 230 ppm or 460 lbs/acre
- Hydrogen (H) = 10 ppm or 20 lbs/acre
The ratio of the amounts of the different cations absorbed on the soil colloids, called the Percent Base Saturation, is an important factor affecting the soil condition and availability of mineral nutrients to plants (14, p 226).
The optimum balance for Percent Base Saturation of each of the cations for most fertile soils is approximately:
- Calcium = 60-75%
- Magnesium = 10-20%
- Potassium = 3-5%
- Sodium 0.5-5%
- Hydrogen 10-15% and
- Other Cations = 5% (including many of the trace elements), though most soils vary considerably from this, and some types of plants may tolerate or prefer different balances (1, p 6).
Total exchange capacity (TEC) actually includes both CEC (negatively charged ions) and anion exchange capacity (positively charged ions) of the soil. TEC is sometimes used interchangably with CEC. The cation or base exchange capacity defines the ultimate limit of soil fertility in respect to available cations (14, p 226). To exceed this limit by the addition of excessive amounts of high analysis mineral fertilizers may cause mineral imbalances and tie-ups and result in a soluble salt content detrimental to the soil life, plants and/or animals eating the plants (12i, p 165-71).
Soil pH
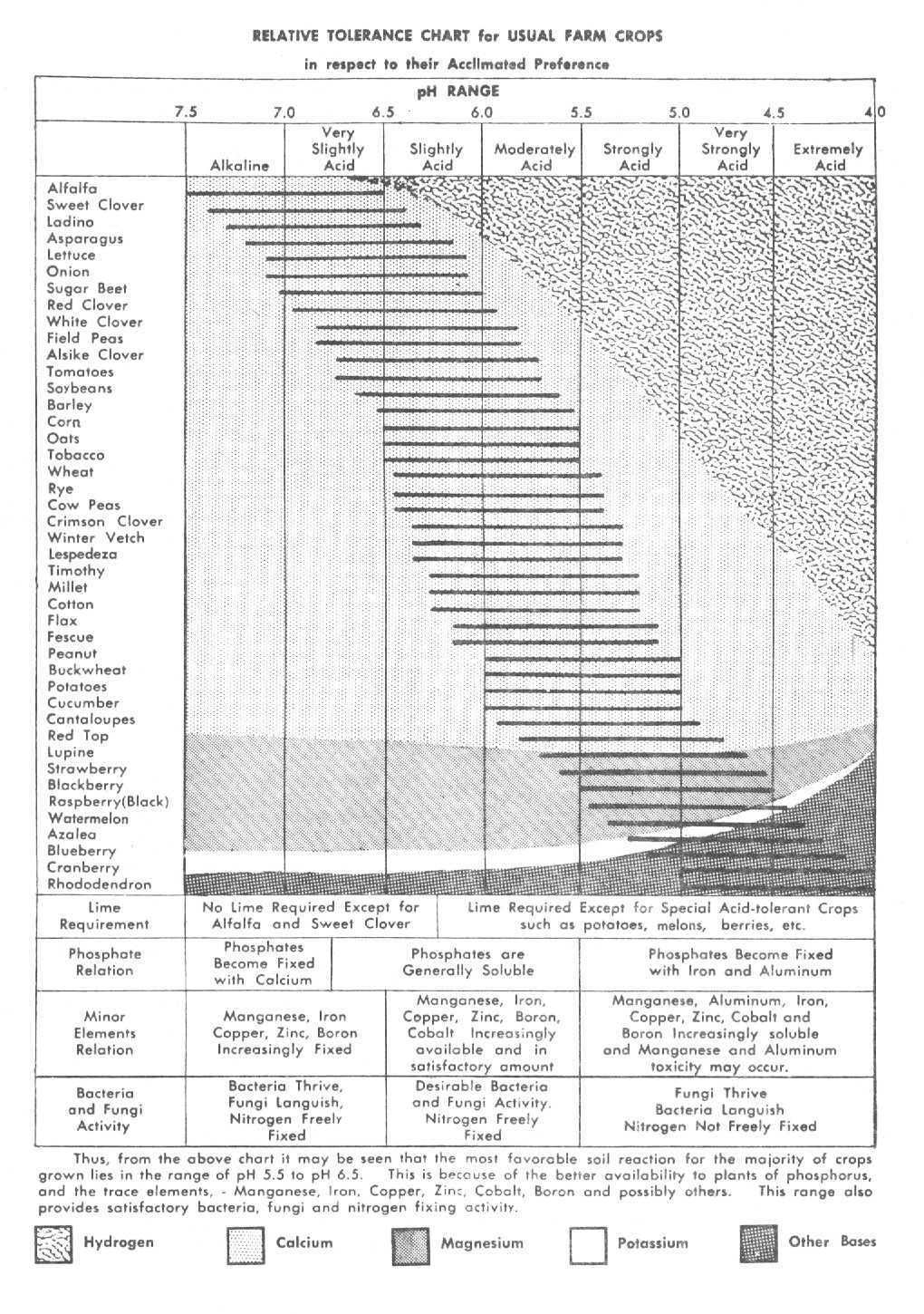 The soil pH is a measure of the soil's hydrogen ion concentration or saturation on the soil colloids (12j, p 67-71). The lower the pH, the higher the hydrogen saturation (more acid). The higher the pH, the higher the level of cation saturation (more alkaline bases) and the lower the percentage of hydrogen saturation. The most desirable range of soil pH for highest plant nutrient availability for most crops is from about 6.5 to 7 (4, p. 290). The common range of soil pH is between 4 and 8. Plants vary considerably in their ability to tolerate various extremes in soil pH. Tolerance is increased by the higher buffering capacity in soils with higher levels of OM.
The soil pH is a measure of the soil's hydrogen ion concentration or saturation on the soil colloids (12j, p 67-71). The lower the pH, the higher the hydrogen saturation (more acid). The higher the pH, the higher the level of cation saturation (more alkaline bases) and the lower the percentage of hydrogen saturation. The most desirable range of soil pH for highest plant nutrient availability for most crops is from about 6.5 to 7 (4, p. 290). The common range of soil pH is between 4 and 8. Plants vary considerably in their ability to tolerate various extremes in soil pH. Tolerance is increased by the higher buffering capacity in soils with higher levels of OM.
The purpose of adding mineral fertilizers to the soil should basically be to fill the soils CEC with the proper balance of cations (Ca, Mg, K and trace elements) and to provide needed anionic elements (N, P, S) that may be deficient. Over fertilization causing mineral imbalances and toxicities is a problem that should be avoided, particularly on soils with low CEC (13, p 16).
Soil Life
Return to Contents
One of the most important factors in building and maintaining soil fertility is the multitude of living organisms within the top few inches. Soil is not soil until it is alive (14, p 224). The life in the soil is a most important part and the real basis of renewable and sustainable soil fertility. No amount of chemical plant food mixed with dead, finely ground rock minerals can produce the equivalent and efficiency of living soil.
The host of bacteria, protozoa, fungi, algae, actinomycetes, nematodes, earthworms, mites, insects, etc. are the specialists responsible for building and maintaining an ideal environment for plants from the dead inert parent rock minerals and organic debris (12d, p 147-65). One gram of soil (1/4 teaspoonful) may contain as many as one million protozoans, twenty million bacteria, one million fungi, and eight hundred thousand algae. Living organisms in most soils may range from 1,000 to 10,000 lbs/acre. 10,000 lbs/acre in a highly fertile soil is like having 10 cows per acre producing manure as fertilizer. The supply of available nitrogen, phosphorus, sulfur and many other plant foods is dependent on the metabolic cycles of these microorganisms. 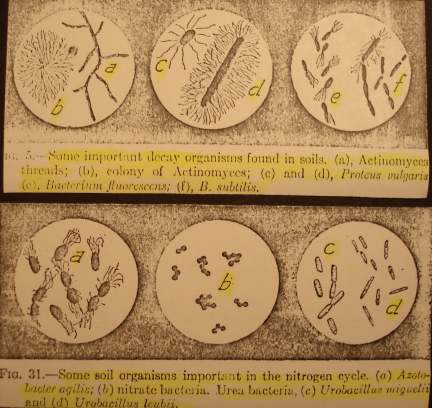
The soil life may be observed by various laboratory techniques, but the effects that result from their activities is readily measured or defined in terms of soil condition and crop response (14, p 225). The presence and activity of earthworms in the soil is one visible indicator that soil conditions are favorable for the soil's microscopic life (12d, p 164). These soil organisms require a proper environment, which includes an ample food supply, including air, water, organic matter and mineral balance.
The types and numbers of organisms present in a soil will be determined largely by climatic conditions such as temperature and moisture levels and soil management practices including tillage, irrigation and fertilization and chemical practices followed. Excess moisture may cause anerobic conditions causing denitrification (loss of nitrogen). Parasitic and pathogenic types often increase under tillage and management practices using excessive and imbalanced chemical fertilizers (especially excess nitrogen) and pesticides that deplete organic matter reserves or otherwise create conditions unfavorable to the beneficial organisms (12e, p 338).
It is important to understand that soil microorganisms have first choice of the available nutrients (14, p 227) and can cause crop deficiencies even when fertilizeres have been applied. The plants may take up only what soil microbes leave or make available. First consideration in any fertilization program should be given to feeding the soil and soil life and balancing the soil's colloidal exchange capacity.
Organic Matter And Humus
Return to Contents
The importance of organic matter (OM) residues to soil fertility is manifold. Organic matter is absolutely vital as the food supply necessary to maintain the life and activities of the soil organisms. The kinds and amounts of residues provided will largely determine the types, numbers and activities of organisms in the soil. Low nitrogen residues activate nitrogen fixing organisms to utilize atmospheric nitrogen for digesting and converting the residues to humus, thereby increasing total nitrogen in the soil. Whereas excess available nitrogen in the soil, such as from fertilizers, stimulates denitrifying organisms to convert it to atmospheric nitrogen, thereby causing a loss of nitrogen in the soil.
As microbial enzymes decompose organic matter, nutrients are released and many by-products are formed (12f, p 151-57). Some of these, such as amino acids, vitamins, enzymes, antibiotics, hormones, growth regulators (such as auxins, cytokinins, gibberellins), etc. are made available to growing plants and are important to their health and production and resistance to disease and insects (6, p 229-30; also 7, p 213-17). Microbial breakdown of plant cellulose to humus and sugars increases water absorption in soils and feeds other organisms and plants.
A key product of organic decomposition, HUMUS is very important for its properties as a soil conditioner, soil colloid, activator and storehouse for many of the mineral and non-mineral elements. As a soil conditioner, humus improves the tilth and structure of soils that are too heavy (tight clay) and those that are too light (sandy) by bonding the fine soil particles into secondary structural units called soil aggregates. These soil aggregates produce an ideal crumbly granular structure with a combination of both large and small pore spaces which provide for good water absorption, holding capacity and drainage, and good aeration.
As a soil colloid, humus (with a CEC of 100 to 300 ME) also greatly increases the soil's buffering capacity and total exchange capacity, and consequently the ability of the soil to retain and supply the positively charged plant nutrients (cations) such as calcium, magnesium, potassium, sodium, ammonium, iron, zinc, copper, manganese, cobalt, etc., and negatively charged anions of nitrogen, sulfur and phosphorus (12c, p 156; also 12f, p 156; & 14, p 226 & 4, p 93-94). Increasing soil humus is the best way to increase the soil's fertility potential.
Available Nitrogen (N)
Return to Contents

Air contains about 78% nitrogen, but plants cannot use this source unless it is converted to usuable forms by soil microbes or other means. Humus contains more than 95% of the soil's total nitrogen, 5 to 60% of the total phosphorus reserve and up to 95 to 98% of the total sulfur (3, p 40-44; also 4, p 118).
Humus is about 5 to 6% nitrogen and 50 to 60% carbon. An acre of soil 6 inches deep weighs about 2,000,000 lbs. A 1% level of organic matter (humus) in the top 6 inches is approximately 20,000 pounds per acre, and will contain a nitrogen reserve of about 1,000 to 1,200 pounds. A 5% level of organic matter will contain a nitrogen reserve of about 5,000 to 6,000 pounds per acre. However, none of this reserve of soil nitrogen is available to plants until it is released by the activity of the soil microorganisms.
The rate at which nitrogen is released will be determined by the the soil type, temperature, moisture, aeration of the soil, mineral balance, pH, carbon-nitrogen ratio and other factors affecting the activity and populations of the soil microorganisms. The estimated rate of nitrogen release (ENR) occurring during the growing season will generally range from 1.25% to 6% depending upon the above factors (12g, p 93). If 4% of the nitrogen reserve is released slowly during the growing season, this would provide 40 to 48 pounds of nitrogen per acre to the crop in a soil with a 1% level of organic matter, and 200 to 240 pounds per acre in a soil with a 5% level of OM. There will be little to no loss of nitrogen provided in this manner.
Proper management and continual replenishment of the soil's organic matter reserves is the single most important responsibility in building and maintaining good soil fertility and tilth (8, p 2-5,9,11,88). Failure to do this has been the major cause of the decline in productivity of soils throughout the world (see the articles, "World Crisis in Agriculture" and "Conquest of the Land Through 7,000 Years").
Many civilizations throughout history have failed to recognize the value of organic residues to provide energy for the work of microbes in the soil, to form humus for improving soil tilth and for making minerals and nitrogen available for crop production. Consequently a common practice has been to simply burn them off to eliminate a "trash" problem and control weeds and plant diseases. This actually increases weed and disease problems in the long run increasing dependency on pesticides and also adds to the greenhouse gases, which some scientists say is causing global warming.
The dollar value of OM residues, at current prices, left from a 50 bushel wheat crop (approximately 2 tons/acre) is easily calculated
- Energy = to 200 gal. of oil @ $70.00/40 gal. barrel = $350.00
- Nitrogen (Urea @$455/ton) = 25 lbs = $12.36
- Phosphate (Super P2O5 @ $515/ton) = 6 lbs = $3.43
- Potash (KCl of K2O @ $345/ton) = 60 lbs = $17.30
- Total value = $383.09 - Plus
- Carbon, the main component of humus = 2,000 lbs. The practical value is in better tilth resulting in increased water absorption and retention in the soil and requiring less power and energy for tillage. This does not include other benefits such as increasing the availability of soil minerals or the fixation of atmospheric Nitrogen. In an on-the-farm research study we did on 30 farms in Iowa in 1982, we found an average increase in total notrogen in the soil of 440 lbs/acre in one season using biological soil management to improve OM residue decomposition verses the regular chemical program (see "Potential of Soil Biotechnology for Profitable & Sustainable Agricultural Systems" Table I).
Hunger Signs in Plants
Return to Contents
 The health and productivity of plants are good indicators of the condition and needs of the soil. However, many other factors including variations in temperature, moisture, light, pesticide applications (especially herbicides), poor plant genetics and lowered resistance to disease and insect damage can also cause symptoms such as decreased growth and production, loss of green color, etc.
The health and productivity of plants are good indicators of the condition and needs of the soil. However, many other factors including variations in temperature, moisture, light, pesticide applications (especially herbicides), poor plant genetics and lowered resistance to disease and insect damage can also cause symptoms such as decreased growth and production, loss of green color, etc.
A growing crop will need only a small amount (a few lbs/acre) of nutrients per day, which increases at maximum growth stages. The crop growth cycle and needs corresponds to the biological cycle and release of nutrients in the soil. The practice of putting all the fertilizer nutrients on before planting is usually very wasteful as much of it may be lost by leaching or chemical tieup before the crop can use it.
Lack of water may also cause inadequate uptake of nutrients from the soil and plant deficiencies and/or sometimes toxicities in crops. Different kinds of plants often show somewhat different symptoms to deficiencies. Following are some of the deficiency symptoms commonly seen in many plants.
Common Plant Deficiency Symptoms
Nitrogen (N):
1. A yellowish-green color.
2. A distinctly slow and dwarfed growth.
3. Drying up or "firing" of leaves, which starts at the bottom of the plant, proceeding upward. In plants like grains and grasses(9) the firing starts at the tip of the bottom leaves and goes down the center or along the midrib.
Excess nitrogen may cause excessive growth, deficiencies of other elements, weakened resistance to diseases and insects, "lodging" in grains and nitrogen toxicities in feed and animals eating on it.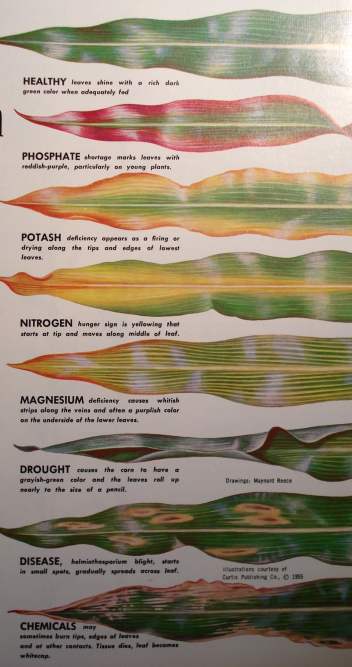
Phosphorus (P):
1. Purplish leaves, stems and branches.
2. Slow growth and maturity, stunting of plants and spindling of roots and tops, small slender stalk in
grass, lack of stoollng in small grains.
Excess phosphorus in chemical forms may combine with and cause deficiencies of trace minerals such as zinc.
Potash (K - Potassium):
1. Mottling, brown top, purplish spotting, streaking or curling of leaves, starting at lower levels.
2. Lower leaves scorched or burned on margins and tips. These dead areas may fall out, leaving ragged edges. In grains and grasses, firing starts at the tip of the leaf and proceeds down from the edge, usually leaving the midrib green.
3. Premature loss of leaves, reduced resistance to cold and other adverse conditions and disease.
4. Plants like grain falling down before mature due to poor root development.
Excess potassium fertilizers in chemical forms have been shown to cause excessively high levels of potassium in plants and sudden death in livestock, especially on low calcium soils.
Calcium (Ca):
Young leaves just beginning to bud become "hooked" In appearance and die back at tips and along margins.
2. Young leaflets of terminal bud may have light green band along margin or be chlorotic.
3. Leaves have a wrinkled appearance, In some cases young leaves remain folded.
4. Root tips die and sluff off with formation of bulb-like enlargements on remaining tips.
Excess calcium may cause molybdenum toxicity and copper deficiency in livestock being fed feeds grown on over limed soils.
Magnesium (Mg):
1. Loss of green color, beginning between ribs.
2. Leaf tissue between veins and along edges turns reddish purple.
Excess magnesium causes poor structure and hardening of soils.
Sulfur (S):
Symptoms resemble those of nitrogen deficiency.
These hunger signs are only a general guide to deficiency problems and should not be used as the only determnation of what and how much fertilizer should be applied, because in many cases deficiency symptoms may be similar to toxicity symptoms.
Soil Sampling Procedures
Return to Contents
The steps for obtaining an accurate analysis of a soil begin with sampling the field. Samples must accurately represent the field being tested. The accuracy and value of the laboratory analysis can be no better than the sample tested. Test results from poorly taken samples may actually be misleading. The following suggestions are given as a guide toward proper sampling.
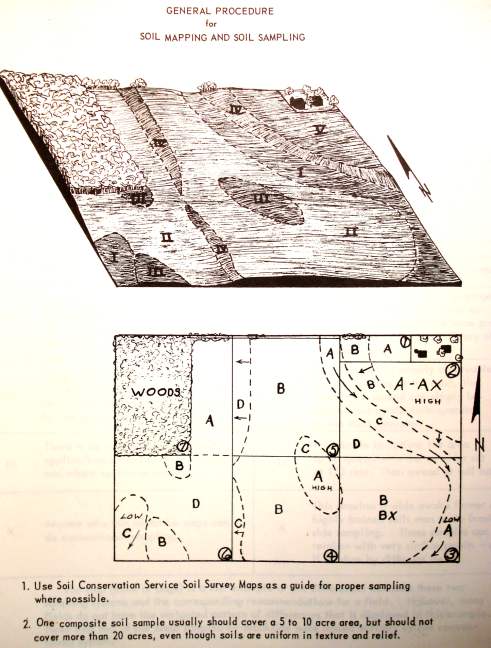
1. A soil map should be made of the field to be sampled as a permanent record for future reference and use in management. A soil survey map with soil types and topography markings made by the Soil Conservation Service is excellent for delineating proper sampling areas. Divide fields according to soil types and conditions, past crop and fertilizer treatments, etc., and take samples from each area large enough to manage separately. Composite samples should not include more than 20 acres under most circumstances.
2. Avoid sampling unusual areas or spots such as old fence lines, dead furrows, eroded spots, fertilizer spots, field depressions, etc., as these areas will not be representative of the whole field. Such areas should be sampled separately if tests are desired.
3. Individual samples can be taken with a spade, sampling tube or auger. If a spade is used, be sure the sample is uniform in profile to represent the soil from the surface to the depth being sampled. The top 6 to 8 inches is commonly sampled, but samples should generally be taken from the soil surface to a depth including the area of major feeder roots of the crops being grown. Subsoil samples and other special samples desired should be taken according to the specific needs. Lab intrepretation of soil test results is based on an average of a 6 inch sample depth. For deeper samples different calculations will need to be made so soil depth must be included with the samples.
4. Collect an adequate number of individual samples (ie. 10 to 15) of uniform depth to represent each area and mix them thoroughly in a clean pail to make a composite sample to send to the lab for testing.
5. Record sample locations on a map to help you or your consultant properly interpret the tests and make proper recommendations.
6. Package and label samples correctly to avoid breakage and mix-ups on samples. Include adequate information such as name, field number, sample identity, depth of sample, date, crops being grown, etc. with the samples and send to a reputable testing laboratory for reliable results. Do not send wet samples as changes will occur in the soil that will change test results.
7. Keeping complete records on crops grown over the past seven years, the type of soil in each field, the fertilizer treatments the soil has had, and all soil sampling and test results can be helpful in planning future cropping and sustainable soil management needs.
Soil Tests
Return to Contents
The tests that should be run on the soil samples will depend on the purpose for testing. For sustainable planning and management of the soil, the following tests should be requested for determining the fertilizer and management needs of the soil:
- 1. Percent of organic matter and estimated nitrogen release.
- 2. Cation exchange capacity.
- 3. pH.
- 4. The major exchangable cations including: calcium, magnesium, potassium, sodium and hydrogen in parts per million or pounds per acre, and the percent base saturation of each.
- 5. The available nitrogen as nitrates and ammonium.
- 6. The available phosphorus and sulfur.
- 7. A one time test of total mineral reserves is recommended to determine the long term mineral maintenance needs of the soil.
- 8. Other information that may be needed, depending on specific problems. The trace elements are not generally tested until the major elements are in balance because an imbalance of major elements can cause a tie-up of minor elements that may be released if the ratio of major elements is first corrected .
Soil Test Results & Recommendations
Return to Contents
Ideally, growers should learn to intrepret the soil test results and make recommendations for their own fields because they have the most experience and knowledge of the fields and management history. Otherwise it would be best to use qualified agricultural consultants having knowledge and experience of the local area and farming practices and conditions to help intrepret and make fertilizeer and management recommendations.
Soil test results giving only low, medium or high readings are generally unreliable. Kit tests giving specific levels of nutrients may be useful, but low, medium and high ratings of the cations are not reliable without knowing the CEC and Percent Base Saturation.
The effective use of laboratory tests as a basis for fertilizer recommendations requires that laboratory results be related to crop response obtained from research with fertilizer applications in the field (10, p 65). Soil test results should be analyzed and correlated with previous cropping and fertilizing practices and results, weather conditions, etc. Field research must consider all of the various soil, climate and management factors that have significant influence on crop yield and response to fertilizers.
The level of organic matter and the cation exchange capacity are the two major factors that should be used to determine the soil's sustainable management and fertilizer needs (12a, p 180). Soils with lower levels of organic matter, less than 3%, need to be managed in a manner to continually increase it. For some soils and climates, this may mean keeping it in permanent cover (such as pasture) unless large amounts of organic matter residues are available to increase it. Soils having a low CEC, less than 7 ME, should also be kept in permanent cover unless sufficient organic fertilizers are available.
Fertilizer recommendations should be determined from the results of the soil analysis, and the availability of proper materials, and the crop requirements for crop and yield goal (see Table: Nutrient Requirements of Crops.
The soil needs for cation minerals (Ca, Mg and K) should be determined mainly by the soil pH, CEC, and Percent Base Saturation of the major cations according to the information mentioned above.
Fertilizer Materials for Correcting Soil Deficiencies & Imbalances
Return to Contents
I. Materials for correcting soil deficiencies and imbalances:
- Composts, animal manures, crop residues, cover crops, biological inoculants, etc.
- Liming materials for low pH soils - High Calcium Lime, Agricultural Lime, Dolomite Lime, Gypsum (Calcium Sulfate), etc.
- Acidifying materials for high pH soils - Organic matter, Sulffur (flowers of), Ferric Sulfate, Ferrous Sulfate, Calcium Sulfate (Gypsum), etc.
- Rock Phosphate is a good source of Phosphorus, Calcium and trace minerals, especially when finely pulverized at about 200 mesh.
- Potash - Potassium Sulfate (K2SO4), Muriate of Potash (KCl), K-Mag (or Sul-Po-Mag - K2SO4.MgSO4), Wood ash is also a good source of K and Ca, etc.
- Trace Elements -
- Nitrogen - Legume cover crops, manures and compost. For supplement to low Nitrogen crop residues and compost piles, apply 1 lb of N/100 lbs of residues (100 lbs of Ammonium Sulfate contains 20 lbs on N is suficient for 1 ton of residues).
II. Materials for Correcting pH and Cation balance:
- Low CEC soils (sandy) - Use coarser grade materials in larger amounts to reduce losses by leaching and for longer lasting effects.
- Low pH due to Low Calcium - Use High Calcium Lime or Ag Lime.
- Low pH due to low Calcium and Magnesium - Use Dolomite Lime or Dolomite and Ag Lime.
- Low pH due to low Ca, Mg, and K - Use Ag Lime and K-Mag or Dolomite Lime and Potash or K-Mag or other combinations.
- Low pH with Low Ca and High Mg, K or Na - Use High Calcium Lime to raise the pH above 6 then use Gypsum to lower the Mg, K or Na.
- Medium pH with Low Ca and P - Use Rock Phosphate, Add Lime if pH is below 6.
- Medium or High pH with Low Ca and High Mg, K, or Na - Use Gypsum and High Calcium Lime if needed.
- High pH with Normal or High Ca, Mg, Na - Use Organic matter, Sulfur, Ferric Sulfate, etc.
- Humus is effective for correcting High or Low pH problems when used with the correct minerals.
- Always be practical - Use Common Sense.
Soil & Feed Testing Laboratories & Consulting Services
Return to Contents
The following agricultural analytical labs and consulting services are some I have used that have proven to be most helpful and accurate.
- BROOKSIDE LABORATORIES, INC.
308 S Main Street, New Knoxville, OH 45871,
(419) 753 2448, Website: www.blinc.com
- MIDWEST LABORATORIES, INC.
13611 "B" Street, Omaha, NE 68144
(402) 334-7770, Website: www.midwestlabs.com
- A&L Analytical Laboratories, Inc.
2790 Whitten Road,
Memphis, TN 38133 - and other locations.
800-264-4522 901-213-2400
Website: www.allabs.com
- A & L Western Laboratories, Inc.
1311 Woodland Avenue Suite 1,
Modesto, CA 95351
Phone: (209) 529-4080
Website: http://www.al-labs-west.com/
- A&L Great Lakes Labatories
3505 Conestoga Drive, Fort Wayne, IN 46808
(260) 483-4759, Website: http://www.algreatlakes.com/
- A&L Plains Agricultural Laboratory, Inc.,
Lubbock, TX 79408-1590
(806) 763-4278 Fax (806) 763-2762
Website: http://www.al-labs-plains.com/
- U.S.D.A. SOIL CONSERVATION SERVICE (SCS), now NRCS
Website: http://soils.usda.gov/
For survey and consultation on soil types and capabilities for best land use and conservation practices:
Offices and agents located in many counties of various States enrolled as soil conservation districts.
- KINSEY AGRICULTURAL SERVICES. INC.
297 County Highway 357
Charleston, Missouri 63834
(573) 683-3880, Website: www.kinseyag.com
- Most states provide some testing and diagnostic services and have extension agents in local county areas. I have found that the tests from many State analytical testing labs to to be of questional value.
- Various other commercial testing companies, agricultural consulting companies and fertilizer and feed companies, etc. may provide analytical services. I have found that tests from many commercial fertilizer companies to be of little value except to sell their fertilizers.
The information presented on soils and soil testing should be helpful in evaluating the testing services of various labs.
Bibliography
Return to Contents
1. Albrecht, William A. “Soil Reaction (PH) and Balanced Plant Nutrition.” Unpublished document, Department of Soils, University of Missouri, December, 1967.
2. Alexander, M. "Introduction to Soil Microbiology," New York: John Wiley & Sons, Inc., 1967.
3. Bremner, J. M. and Tabatabai, M. A. “Distribution of Total and Available Sulfur in Selected Soils and Soil Profiles,” Volume 64, No. 1 (Jan—Feb, 1972), PP. 40—44.
4. Donahue, R. L. "Soils: An Introduction to Soils and Plant Growth," 2nd ed. Englewood Cliffs, New Jersey: Prentice-Hall Inc., 1965.
5. Epstein, Emanuel. "Mineral Nutrition of Plants: Principles and Perspectlves," New York: John Wiley and Sons, Inc., 1972.
6. Gray, T. R. G. and Parkinson, D., ed. "The Ecologv of Soil Bacteria, an International Symposium," Canada: University of Toronto Press, 1968.
7. Kononova, M. M., "Soil Organic Matter," 2nd edition. Oxford: Pergamon Press, 1966.
8. Lundell, Cyrus Longworth, "Agricultural Research at Renner," 1944-1966. Menasha, Wisconsin: George Banta Company, Inc., 1967.
9. Nelson, Warner L and Tisdale, Samuel L., "Soil Fertility and Fertilizers," 2nd ed. NewYork: MacMillan Co., 1966.
10. Olson, R. A. ed., "Fertilizer Technology and Use," 2nd ed. Madison, Wisconsin: Soil Science Society of America, 1971.
11. Sprague, Howard B. ed., "Hunger Signs in Crops," 3d ed. New York: David McKay Company, 1964.
12. Stefferud, Alfred, ed. "Soil," The United States Department of Agriculture (USDA) 1957 Yearbook of Agriculture. Washington, D. C.: The United States Government Printing Office, 1957.
12a. John I. Hanway and Frank G. Viets, Jr., “How to Determine Nutrient Needs,” Soil, The USDA 1957 Yrbk of Agriculture.
12b. Roy W. Simonson, "What Soils Are," Soil, USDA 1957 Yrbk of Agriculture.
12c. L. A. Dean, "Soil," USDA 1957 Yrbk of Agriculture.
12d. Francis E. Clark, "Living Organisms in the Soil," USDA 1957 Yrbk of Agriculture.
12e. F. E. Clark, I. T. Presley and W. I. Zaumeyer, “Soilborne Plant Diseases,” Soil, USDA 1957 Yrbk of Agriculture.
12f. F. E. Broadbent, “Organic Matter,” Soil, USDA 1957 Yrbk of Agriculture.
12g. Franklin E. Allison, "Nitrogen and Soil Fertility," Soil, USDA 1957 Yrbk of Agriculture.
12h. F. Reitemeier, “Soil Potassium and Fertility,” Soil, USDA 1957 Yrbk of Agriculture.
12i. Firman E. Bear, “Toxic Elements in Soils,” Soil, USDA 1957 Yrbk of Agriculture.
12j. W. H. Allaway, “pH, Soil Acidity, and Plant Growth,” Soil, USDA 1957 Yrbk of Agriculture.
13. Voss, R. D. “Cation Exchange Capacity,” Crops and Soils Magazine, December, 1971. (Reprinted In World Farming, June, 1972 as “Cation Exchange Capacity - How Nutrients Behave in the Soil.”)
14. Wynd, F. L. “Feed the Soil,” The Scientific Monthly, Volume 74, No. 4, April, 1952.
15. Thompson & Troeh, "Soils and Soil Fertility," McGraw-Hill Book Company, 1973.
Copyright ©: 1972 - Allen L. Stout; 2007 - AgroTech Research Group, Inc., & Serf Publishing, Inc.,
Latest Update: 10/18/07
 A thin fragile layer of living Topsoil, varying from a few inches to a few feet thick, covering much of the Earth's surface is all that stands between humanity and starvation. The rise and fall of great civilizations have been determined by how the soil has been managed or exploited (see "World Crisis in Agriculture" and "Conquest of the Land Through 7,000 Years").
A thin fragile layer of living Topsoil, varying from a few inches to a few feet thick, covering much of the Earth's surface is all that stands between humanity and starvation. The rise and fall of great civilizations have been determined by how the soil has been managed or exploited (see "World Crisis in Agriculture" and "Conquest of the Land Through 7,000 Years"). Twenty elements are presently known to be essential for the growth of plants, and the importance of others is beginning to be recognized (9, p 105; also 4, p 4). Not all are required by all plants, but all are necessary to some plants. Carbon, hydrogen, and oxygen are derived from air and water; nitrogen from air and soil; and phosphorus, potassium, sulfur, calcium, magnesium, sodium, iron, manganese, boron, copper, cobalt, zinc, molybdenum, vanadium, chlorine and silicon from the soil (4, p 4). Plants also contain iodine and selenium (elements essential to animals) and many other elements whose functions in plants are not totally known (12c, p 80; also 9, p 533-34; & 4, p 11).
Twenty elements are presently known to be essential for the growth of plants, and the importance of others is beginning to be recognized (9, p 105; also 4, p 4). Not all are required by all plants, but all are necessary to some plants. Carbon, hydrogen, and oxygen are derived from air and water; nitrogen from air and soil; and phosphorus, potassium, sulfur, calcium, magnesium, sodium, iron, manganese, boron, copper, cobalt, zinc, molybdenum, vanadium, chlorine and silicon from the soil (4, p 4). Plants also contain iodine and selenium (elements essential to animals) and many other elements whose functions in plants are not totally known (12c, p 80; also 9, p 533-34; & 4, p 11). 
 The mineral portion consists of sand, silt, clay and larger rock particles and the soluble ionized mineral elements in the soil solution and attached to the soil colloids. The mineral particles give a soil its characteristic texture and serve as a storehouse for most of the soil's mineral reserves. However, very little of these reserves are available to plants. For example, an average acre foot of a silt loam soil may contain 70,000 pounds of total potash, but depending on the mineral types, only 100 to 600 pounds may usually be in a form available to plants (12h, p 101).
The mineral portion consists of sand, silt, clay and larger rock particles and the soluble ionized mineral elements in the soil solution and attached to the soil colloids. The mineral particles give a soil its characteristic texture and serve as a storehouse for most of the soil's mineral reserves. However, very little of these reserves are available to plants. For example, an average acre foot of a silt loam soil may contain 70,000 pounds of total potash, but depending on the mineral types, only 100 to 600 pounds may usually be in a form available to plants (12h, p 101).  CEC is a property of the soil colloids (clay and humus content), which have a negative charge to hold positively charged cations (Ca, Mg, K, Na, H and trace mineral cations).
CEC is a property of the soil colloids (clay and humus content), which have a negative charge to hold positively charged cations (Ca, Mg, K, Na, H and trace mineral cations). The soil pH is a measure of the soil's hydrogen ion concentration or saturation on the soil colloids (12j, p 67-71). The lower the pH, the higher the hydrogen saturation (more acid). The higher the pH, the higher the level of cation saturation (more alkaline bases) and the lower the percentage of hydrogen saturation. The most desirable range of soil pH for highest plant nutrient availability for most crops is from about 6.5 to 7 (4, p. 290). The common range of soil pH is between 4 and 8. Plants vary considerably in their ability to tolerate various extremes in soil pH. Tolerance is increased by the higher buffering capacity in soils with higher levels of OM.
The soil pH is a measure of the soil's hydrogen ion concentration or saturation on the soil colloids (12j, p 67-71). The lower the pH, the higher the hydrogen saturation (more acid). The higher the pH, the higher the level of cation saturation (more alkaline bases) and the lower the percentage of hydrogen saturation. The most desirable range of soil pH for highest plant nutrient availability for most crops is from about 6.5 to 7 (4, p. 290). The common range of soil pH is between 4 and 8. Plants vary considerably in their ability to tolerate various extremes in soil pH. Tolerance is increased by the higher buffering capacity in soils with higher levels of OM.

 The health and productivity of plants are good indicators of the condition and needs of the soil. However, many other factors including variations in temperature, moisture, light, pesticide applications (especially herbicides), poor plant genetics and lowered resistance to disease and insect damage can also cause symptoms such as decreased growth and production, loss of green color, etc.
The health and productivity of plants are good indicators of the condition and needs of the soil. However, many other factors including variations in temperature, moisture, light, pesticide applications (especially herbicides), poor plant genetics and lowered resistance to disease and insect damage can also cause symptoms such as decreased growth and production, loss of green color, etc.
